Highlights
A germ cell‐specific ageing pattern in otherwise healthy men (09/2020)
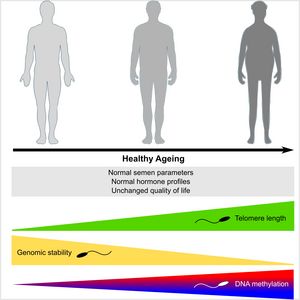
Life‐long sperm production leads to the assumption that male fecundity remains unchanged throughout life. However, recently it was shown that paternal age has profound consequences for male fertility and offspring health. Paternal age effects are caused by an accumulation of germ cell mutations over time. However, molecular patterns of ageing in germ cells and their impact on DNA integrity have not been studied in detail. To assess the effects of ‘pure’ ageing on male reproductive health and germ cell quality, we assembled a cohort of 198 healthy men (18–84 years) and evaluated a variety of end points. While sperm production and hormonal profiles were maintained at physiological levels over a period of six decades, we identified a steady increase of telomere length in sperm, a sharp increase in sperm DNA instability and sperm DNA methylation changes in 236 regions. In conclusion, human male germ cells present a unique germline‐specific ageing process, which likely results in diminished fecundity in elderly men and poorer health prognosis for their offspring.
Does the FSHB c.-211G greater than T polymorphism impact Sertoli cell number and the spermatogenic potential in infertile patients? (02/2020)

Our group is working on the impact of c.-211G>T FSHB single nucleotide polymorphism (SNP) on spermatogenesis. We and others have recently shown that this SNP is strongly associated with lowered testicular volume, reduced sperm counts and decreased FSH levels in patients carrying one or two T-alleles. However it was not clear to which extent Sertoli cell (SC) number, Sertoli cell workload (SCWL) and thereby spermatogenic potential is affected. In the current study we demonstrated that neither SC number nor SCWL is significantly different among different genotypes of the -211G>T FSHB SNP (see figure). Thus, the spermatogenic potential is maintained independent of the SNP genotype. The previously observed clinical phenotype might be caused by a hypo-stimulated spermatogenesis and not due to a decreased SC number. Our findings support the concept for a putative treatment of infertile men with FSH to stimulate spermatogenesis further.

