Our Research


‘We address how enteric pathogens differ from the harmless colonizers of our intestine and how they promote intestinal infections and cause disease.’


Reprogramming of Yersinia during infection to adapt to host niches
Enteropathogenic Yersiniae undergo different infection phases: Initial Infection Phase (colonization) - Acute Infection Phase (host defense) - Persistent Infection Phase (immune evasion). We investigate which pathogenicity factors and virulence-associated processes are expressed during the individual infection phases and how their expression is initiated and reprogrammed in response to the environmental conditions experienced in the different tissues and infection phases. For this purpose, we use RNA-seq based technologies (e.g. tissue dual RNA-seq to identify genes and that are induced/repressed in the pathogen and the host during the course of the infection (Avican et al. PLoS Pathogens 2015, Nuss et al. 2017 PNAS, Schmühl et al. 2019 mSystems, Kusmierek et al. 2019 PLoS Pathogens).

Reprogramming of Yersinia virulence factor expression for colonization to host defense upon transfer from the gut lumen into subepithelial lymphoid tissues.

Tissue dual-RNA-seq analysis to study global spatial-temporal control of Yersinia virulence (A) and identified global regulatory network including regulatory RNAs of Y. pseudotuberculosis (B).
Analysis of networks and regulatory factors important for virulence
Bacterial gene control is a multifaceted and highly dynamic process which is administered by complex global regulatory networks in response to environmental cues. It is well-known that pathogens can adopt and switch between alternative phenotypes (bistable, heterogeneous expression) which allows them to adapt to rapidly changing environments (bet hedging). These complex control processes involve the interplay of various regulatory factors and RNA elements, such as RNA thermometer and small regulatory RNAs. We study the molecular mechanisms underlying virulence gene control in enteropathogenic Yersiniae with a focus on sensory and regulatory RNAs and small proteins using different techniques such as RNA-seq, RIP/CLIP-seq or Ribo-seq (Nuss et al. 2015 PLoS Pathogens, Nuss et al. 2016 PLoS Pathogens, Wang et al. 2016 Science; Kusmierek et al. 2019 PLoS Pathogens, Knittel et al. 2020 PLoS Pathogens, Twittenhoff et al. 2020, PLoS Pathogens).

Bistable expression of the virulence regulator RovA of Yersinia pseudotuberculosis in a bacterial colony in vitro (left upper panel) and within infected lymphoid tissues (left lower panel). Illustration of heterogeneous expression visuable with rovA-gfp fusions (green) in mCherry-labelled (red) bacteria within the lymphoid tissues.

Role of type III secretion and injected effectors during infection
An aim of our work is the analysis of virulence factors important for the acute and persistent infection on the molecular, cellular and organismic level. A special focus is the type III secretion system (T3SS) with which the bacteria inject anti-phagocytic and anti-inflammatory effector proteins (Yops in Yersiniae) into host (immune) cells to evade and inhibit the immune response.

T3SS needle structure (injectisome) exposed on the surface of Y. pseudotuberculosis by transmission and scanning microscopy. Scheme illustrated T3SS-mediated injection of the Yop effector proteins which is enhance by the secreted CNFY toxin.

Many of the T3SS effectors interfere with important inflammatory pathways activated by NFkB and MAPKinases. Some of the effectors have also the capacity to penetrate host cell membranes independent of the T3SS (‘cell-penetrating effector proteins’ - CPE), e.g. YopM, IpaH9.8. and modulate the host cell signalling pathways. We investigate the molecular mechanisms how these self-penetrating effectors pass through host cell membranes. Moreover, we identify the host cell targets and that are manipulated by these effectors (Norworski et al. 2018 Cell. Mol. Life Sci. Norworski et al. 2018 Cellular Microbiology, Grabowski et al. 2017, Virulence).
Structure, function and role of bacterial toxins during infection
Most enteric bacteria produce and secrete toxins (exotoxins) such as the cytotoxic necrotizing factor CNFY in Yersinia. We study the structure and function of toxins and could demonstrate that CNFY constitutively activates Rho-GTPases in host cells. This enhances the translocation of the Yop effectors by the T3SS and amplify their function. It further leads to major morphological changes, e.g. formation of stress fibers and giant multi-nucleated cells (Schweer et al. 2013 PLoS Pathogens, Heine et al. 2018 PLoS Pathogens, Chaoprasid et al. 2021, EMBO J).


Secretion and translocation of the CNFY toxin by Y. pseudotuberculosis with the leads to massive actin cytoskeletal rearrangements, giant multinucleated cells and triggers strong inflammation of the intestinal epithelium.
We also study the role of out membrane vesicles for the import and function of the toxin and investigate their impact on the destruction/lesion formation (Bielaszewska M et al. 2017 PLoS Pathogens).
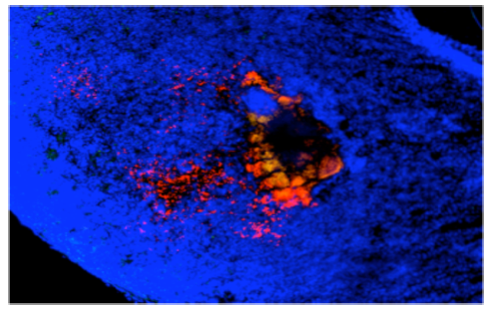
Y. pseudotuberculosis (labelled in red) leads to massive tissue destruction and the formation of large lesions in the gut-associated tissues. The toxin is secreted directly and bound to outer membrane vesicles. Inside the host cells it constitutively activates Rho GTPases and triggers an inflammatory cell death.
We study the role of outer membrane vesicles (OMV) as a highly advanced mechanism for secretion and simultaneous, coordinated and direct delivery of bacterial toxins into host cells. OMVs can be considered as bacterial ‘long distance weapons’ that attack host tissues and help bacterial pathogens to establish the colonization of their biological niche(s), impair host cell function, and modulate the defense of the host. (Bielaszewska M et al. 2017 PLoS Pathogens, Rüter and Bielaszewska 2020 Front Cell Infect Microbiol.).

Outer membrane vesicle from gram negative bacteria. (A) Schematic representation of OMV with cargo. (B) Exemplary electron microscopy pictures of outer membrane vesicles.
Use of bacterial factors for therapeutic approaches
We develop strategies to block bacterial toxin of type III secretion system function without or combined with antibiotic treatments (Mühlen & Dersch 2020 Frontiers Microbiology, Mühlen et al. 2020, Antimicrob. Agents Chemotherapy). We further use bacterial invasion factors, toxins and bacterial effector proteins (‚cell-penetrating effectors‘) and test their use as potential drug delivery tools and anti-inflammatory agents. For instance, we could demonstrate that the CNFY toxins consists of an N-terminal transport module with which cargo proteins such as GFP or the b-lactamase (Bla) can be delivered into the host cells (Chaoprasid et al. 2021, EMBO J). Moreover, we showed that invasin can be used to promote uptake of drug-carrying liposomes. Currently, we investigate the delivery potential of different effectors of pathogenic Yersinia und Shigella species (e.g. YopM, IpaHs) and test their capacity as ´self-delivering´ anti-inflammatory agents (Rüter et al. 2018, Int. J. Med. Microbiol., Rüter & Schmidt 2017, Trends in Biotechnology).

FRET-based analysis of toxin injection into host cells by toxin-fusion proteins with b-lactamase (Bla).

Cell-penetrating effector proteins, the Highway to Cell. (A) Schematic overview of YopM and LPX effector proteins. LPX effector proteins target host cell substrates for ubiquitinylation. Consequent deregulation of the host ubiquitin system favors bacterial proliferation inside the host cell. (B) Cellular distribution of gold-conjugated YopM. HeLa cells were incubated for 1 h with gold-conjugated recombinant YopM and prepared for TEM.

How do enteric bacteria bind and transmigrate through cells
(cell adhesion, invasion and exit strategies)

We analyze how enteric bacteria adapt to the intestinal environment, adhere to and invade intestinal epithelial cells. They use different strategies at different time points of the infection (Cordes et al. 2016 Inflamm. Bowel. Dis., Sadana et al. 2018, J. Biol. Chem., Schiller et al. 2020, Virulence). The structure and function of the individual adhesion and invasion factors and they role during the infection process are investigated.
The internalization of the bacteria is promoted by the induction of a cascade of signaling pathways upon adherence (Baumann et al. 2018, Int. J. Med. Microbiol.).

This leads to actin cytoskeletal rearrangements that enclose the bacteria in a membrane-enclosed vacuole (zipper and trigger mechanism).
The bacteria are liberated from the phagosome upon exit at the basolateral side of the epithelial layer. How they induce the exit process is still unknown and is current under investigation.

We study how bacteria counteract the response of the host immune system when they enter the gut-associated lymphatic tissues. For this we use oral mouse infection models (Derbise et al. 2020, Infection & Immunity, Pisano et al. 2012, Infection & Immunity). Based on our tissue dual RNA-seq study we could identify several host defense mechanisms that are important to eradicate a Yersinia infection (Nuss et al. 2017, PNAS). One strategy is the induction of inflammation and inclusion of the bacteria within the microcolonies in fibrin clots/networks by induction of the coagulation cascade. This triggers the recruitment of neutrophils to the site of infection to eliminate the invaders. The molecular mechanisms driving this response and how the bacteria manage to evade this response in some hosts and induce a persistent infection is currently under investigation. Moreover, we are interested which immune cells are recruited and activated during the different stages of the infection and how they interact with the pathogen (Petzoldt et al. 2017, J. Infect. Dis.. Pasztoi et al. 2017 Cell. Mol. Life Sci.). In this context, we want to understand (i) when and how the bacteria switch from the acute to the persistent phase, (ii) which adaptation and immune evasion strategies are required and (iii) how this changes the host response (Avican et al. 2015 PLoS Pathogens, Heine et al. 2017 PLoS Pathogens).

Reprogramming of the pathogenicity tools set of Y. pseudotuberculosis and the host response during the transition from the acute infection to the persistent infection phase.


