Cancer is a leading cause of mortality within the aging European population and worldwide [http://ec.europa.eu/eurostat/statistics-explained/index.php/]. Therapeutic targeting is hampered by the complexity of the disease, which includes not only molecular changes within the tumor cell itself but also within its microenvironment. Tumor angiogenesis, tumor-stroma interactions, interactions with immune cells, with the extracellular matrix (ECM) and cancer stem cell niches allow for malignant cell survival and promote metastasis, the leading cause for cancer-associated mortality. Notably, glycoproteins substituted with the heparin-related carbohydrate heparan sulfate (HS) have been shown to be dysregulated in malignant diseases, and are known to modulate all of the aforementioned processes of tumor progression4-8. While implicated in the pathogenetic process, pharmacological targeting of many of these HS-proteoglycans (PGs) is hampered by the fact that they are often expressed at high levels by healthy tissue (as in the case of the epithelial PG Syndecan-1), bearing the danger of therapeutic side effects.
Several functions of HS-PGs in tumor progression are modulated by the enzyme heparanase (HPSE), which is barely expressed in adults, but upregulated during tumor progression, inflammation and angiogenesis, thus constituting an excellent drug target. Indeed, HPSE (= HPSE-1, Hpa1), the sole heparan sulfate degrading endoglycosidase, regulates multiple biological activities that enhance tumor growth, metastasis, angiogenesis and inflammation. HPSE accomplishes this by degrading HS and thereby regulating the bioavailability of heparin- binding proteins, priming the tumor microenvironment and mediating tumor-host crosstalk. HPSE expression is enhanced in almost all cancers examined including various carcinomas, sarcomas and hematological malignancies. Numerous clinical association studies have consistently demonstrated that upregulation of HPSE expression correlates with increased tumor size, tumor angiogenesis, enhanced metastasis and poor prognosis. Consequently, HPSE inhibitors used in tandem with chemotherapeutic drugs overcome initial chemoresistance, providing a strong rationale for applying anti-HPSE therapy in combination with conventional anti-cancer drugs (Fig.1).
HEPINIB will provide the groundwork for the development and preclinical testing of rationally designed drugs for novel HPSE-centered anticancer therapy.
Objectives of the project include:
- Development and preclinical assessment of novel HPSE inhibitors
- Targeting HPSE function in tumor-initiating cells
- Targeting of novel HPSE functions in the tumor microenvironment
- Elucidation of the tumor suppressor activity of HPSE2 / Hpa2
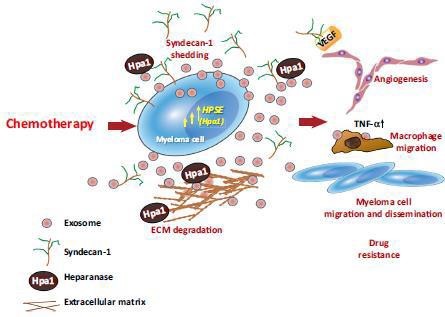
Fig.1. HPSE-carrying exosomes released following chemotherapy facilitate tumor progression10.Treatment of myeloma cells with bortezomib causes a burst of exosome secretion. These exosomes (referred to as chemoexosomes) carry an abundance of HPSE (Hpa) bound to the HS of syndecan-1 present on the exosome surface. Chemoexosomes can transfer HPSE to other tumor cells, thereby enhancing their chemoresistance. HPSE also stimulates syndecan-1 shedding by those cells, facilitating tumor metastasis and angiogenesis. Chemoexosomes may also have VEGF bound on their surface that aids in driving angiogenesis. They also dock with macrophages, stimulating their migration and enhancing their expression of TNF-a. HPSE present on the exosome surface is also available to degrade HS within the ECM, which may liberate tumor-supporting growth factors and facilitate tumor and host cell migration.

