Synapses are specialized intercellular junctions in which cell adhesion molecules connect the presynaptic machinery for neurotransmitter release to the postsynaptic machinery for receptor signalling. Neurotransmitter release requires the presynaptic co-assembly of Ca2+-channels with the secretory apparatus, but little is known about how these components are organized. We have shown over the past years that neurexins are synaptic cell-surface molecules that are required for Ca2+-triggered exocytosis. The most striking feature of mice lacking various neurexin variants is the dramatic reduction in spontaneous and evoked neurotransmission at both glutamatergic and GABAergic synapses. In addition to their role at central synapses, neurexins also affect transmission at the neuromuscular junction, and contribute substantially to Ca2+-triggered release of secretory granules from endocrine cells, calso onsistent with an alteration of dense core vesicle distribution observed in mutant brains.
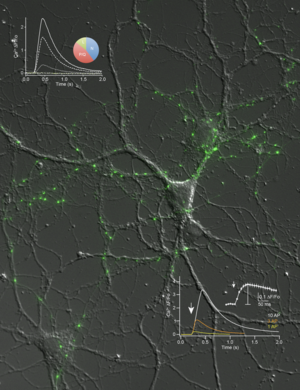
Our current hypothesis maintains that the functional defects in transmission are due to an impaired function or localization of high voltage-activated Ca2+ channels. In support, imaging presynaptic Ca2+ influx with genetically encoded indicators reveals an overall reduction in neurexin-deficient terminals and alterations in the contribution of different Ca2+ channel subtypes (Cav2.1, Cav2.2, etc.). Currently, we investigate the subcellular targeting of neurexins and Ca2+ channel subunits to the synapse, and try to determine the respective molecular determinants and interaction partners involved.

