Organization principles of tissue-resident immune cell networks
Many immune cell subsets (neutrophils, lymphocytes, migratory dendritic cells) are heavily trafficking between organs and inside tissues where they perform fast amoeboid cell movement. Previous work has shown that these fast-migrating immune cells do not require adhesive interactions with the extracellular environment, but rely almost exclusively on cell shape changes driven by the actomyosin cytoskeleton for their movement in interstitial tissues (Lämmermann et al., Nature 2008; Lämmermann et al., Nature 2013; Lämmermann and Kastenmüller, Immunol Rev 2019).
In contrast, other immune cell subsets (e.g. mast cells, resident dendritic cells, macrophages) are tissue-resident and already reside in peripheral tissues or lymphoid organs where they are sessile or slowly migrating. Most of these immune cell subsets form cellular networks of hundreds of individual cells that appear to evenly distribute throughout an entire tissue. To date, we have very limited knowledge about the biological requirements on the single cell and population level in order to form these homogeneous networks. We recently showed that the movement of mast cells (immune cells with important roles during allergy and anaphylaxis) differs fundamentally from the widely applied paradigm of interstitial immune cell migration (Kaltenbach et al., Nat Immunol 2023). We identified a crucial role for integrin-dependent adhesion in controlling mast cell movement and localization to anatomical niches rich in KIT ligand, the major mast cell growth and survival factor. Additionally, our research explores how the cytoskeletal regulation in individual cells influences the formation of immune cell networks and the effector function of the whole cell population. In collaboration with the lab of Angelika Rambold (Max Planck Institute of Immunobiology and Epigenetics; starting in the Institute of Medical Biochemistry, ZMBE, in January 2025), we also investigate the organellar and metabolic pathways that underlie the formation of immune cell networks. By combining in-vivo imaging, mouse genetics and 3D in vitro setups, we seek to identifiy the essential parameters and compensatory mechanisms that immune cells have developed to maintain a fully functional cellular network (Kaltenbach et al., Nat Immunol 2023; Paterson & Lämmermann, eLife 2022).
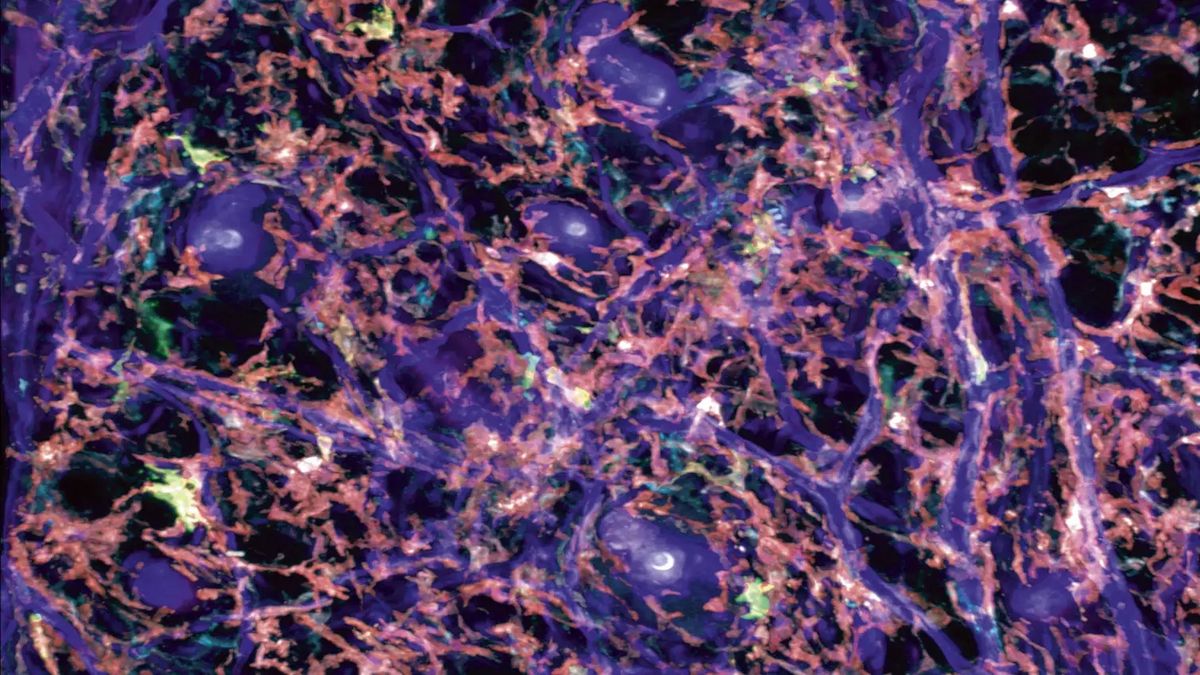
© Tim Lämmermann


