© A. S. Stolle
© B. Grabowski


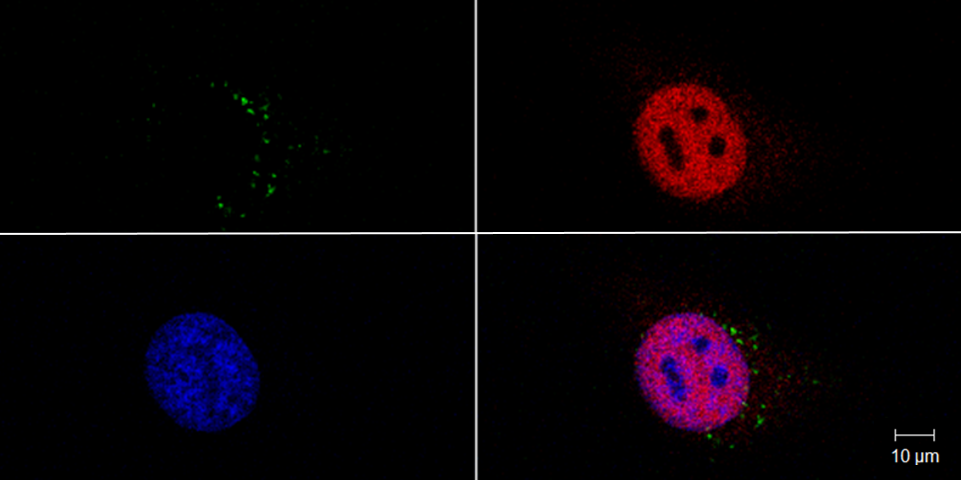
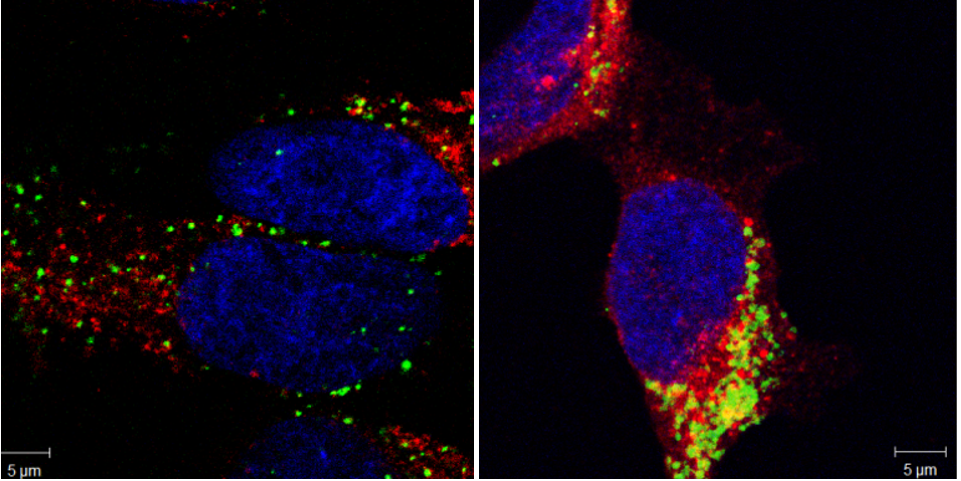
Effektorproteine können unabhängig vom Typ III-Sekretionssystem (T3SS) in Wirtszellen eindringen und so ihren potentiellen Funktionsbereich auch auf zelluläre Barrieren erweitern. Hierzu konnten wir die gesamte Gruppe der bakteriellen Effektoren mit Leucin-reichen Wiederholungen (LPX) als bislang unbekannte zell-penetrierende Effektoren (CPE) identifizieren. Nun wollen wir die Auswirkungen dieser neuen Klasse von CPE auf die Modulation von Immunantworten in Zellkultur- und Tier- Modellen genauer untersuchen und so mögliche therapeutische Anwendungen dieser CPE erforschen.
Forschungsgebiet: Infektionsbiologie
Dr. rer. nat. Christian Rüter (07/2016 - 06/2024)
Prof. Dr. rer. nat. M. Alexander Schmidt (07/2012 - 06/2020)
Projektlaufzeit: Juli 2012 - Juni 2024
Originalartikel
Bäumer N, Scheller A, Wittmann L, Faust A, Apel M, Nimmagadda SC, Geyer C, Grunert K, Kellmann N, Peipp M, Kailayangiri S, Gutierrez Suburu ME, Strassert CA, Schenk M, Greune L, Rüter C, Dersch P, Hartmann W, Rössig C, Neri D, Müller-Tidow C, Schwöppe C, Schliemann C, Khandanpour C, Lenz G, Berdel WE, Bäumer S (2022) Electrostatic anti-CD33-antibody-protamine nanocarriers as platform for a targeted treatment of acute myeloid leukemia. J Hematol Oncol. 15(1): 171. doi:10.1186/s13045-022-01390-5
Bäumer N, Tiemann J, Scheller A, Meyer T, Wittmann L, Suburu MEG, Greune L, Peipp M, Kellmann N, Gumnior A, Brand C, Hartmann W, Rössig C, Müller-Tidow C, Neri D, Strassert CA, Rüter C, Dersch P, Lenz G, Koeffler HP, Berdel WE, Bäumer S (2022) Targeted siRNA nanocarrier: a platform technology for cancer treatment. Oncogene. 41(15):2210-2224. doi:10.1038/s41388-022-02241-w
Faust A, Bäumer N, Schlütermann A, Becht M, Greune L, Geyer C, Rüter C, Margeta R, Wittmann L, Dersch P, Lenz G, Berdel WE, Bäumer S (2022) Tumor-cell-specific targeting of Ibrutinib: Introducing electrostatic antibody-inhibitor conjugates (AiCs). Angew Chem Int Ed Engl. 61(1):e202109769. doi:10.1002/anie.202109769
Rüter C (2022) Delivery of antibiotics by cell-penetrating peptides to kill intracellular pathogens. Methods Mol Biol 2383: 335-345. doi:10.1007/978-1-0716-1752-6_22"
Bielaszewska M, Greune L, Bauwens A, Dersch P, Mellmann A, Rüter C (2021) Virulence Factor Cargo and Host Cell Interactions of Shiga Toxin-Producing Escherichia coli Outer Membrane Vesicles. Methods Mol Biol 2291:177-205
Rüter C, Bielaszewska M (2020) Secretion and Delivery of Intestinal Pathogenic Escherichia coli Virulence Factors via Outer Membrane Vesicles. Front Cell Infect Microbiol 10: 91
Baumann D., Salia H., Greune L., Norkowski S., Körner B., Uckeley Z.M., Frankel G., Guenot M., Rüter C., Schmidt M.A. (2018) Multitalented EspB of enteropathogenic E. coli (EPEC) enters cells autonomously and induces programmed cell death in THP-1 cells. Int. J. Med. Microbiol. 308:387–404.
GomarascaM., MartinsT.F.C., Greune L., Hardwidge P.R., Schmidt M.A., and RüterC. (2017) Bacterium-derived CPP deliver gentamicin to kill intracellular pathogens. Antimicrob. Agents Chemother. 61(4):e02545-16.
Reviews